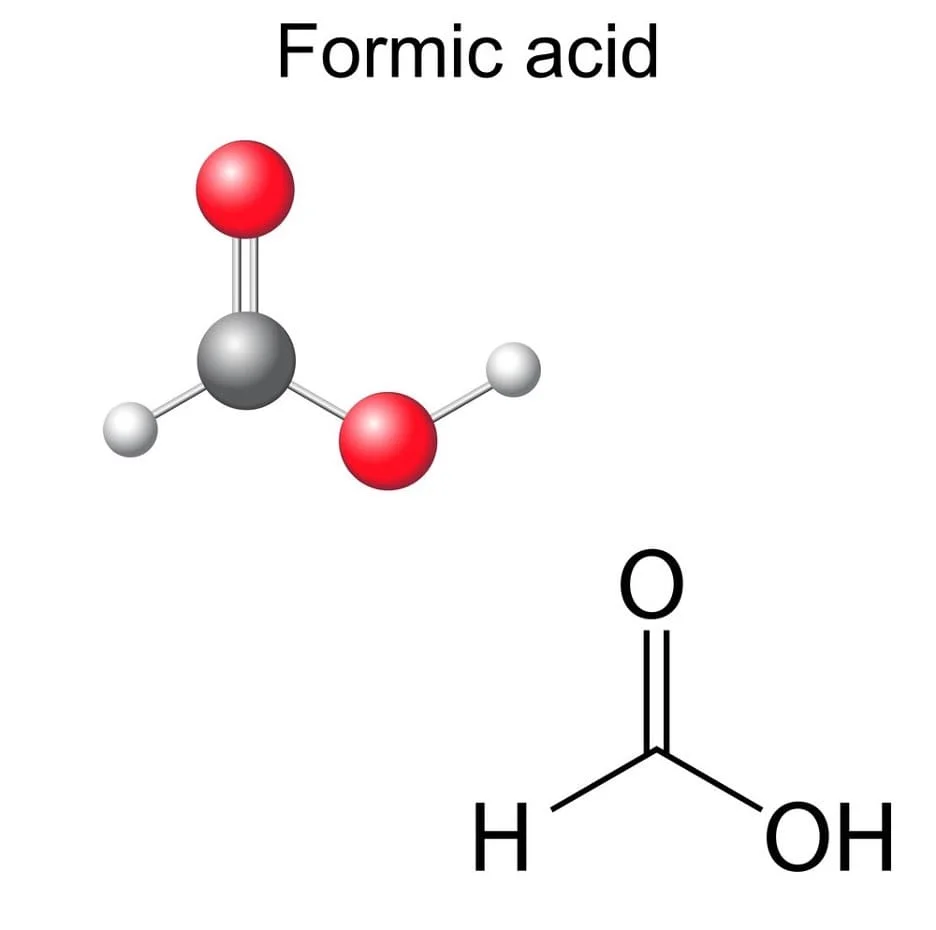
Formic Acid
formic acid:
Formic acid, ant ink, or methanoic acid is systematically the simplest carboxylic acid and has the chemical formula HCOOH. It is an important intermediate in chemical synthesis and is produced naturally. The word “formic” is derived from the Latin word ant, formica, which means distillation of an ant’s body. The ester, salt, and anion of formic acid are called formates. Industrially, formic acid is produced from methanol.

History of formic acid:
Some alchemists and naturalists have known that ant hills give off acidic vapors since the early 15th century. The first person to describe the separation of this substance (by distilling a large number of ants) was the English naturalist John Ray in 1671. Ants secrete formic acid for attack and defense purposes. Formic acid was first made from hydrocyanic acid by the French chemist Joseph Guy Losac. In 1855, another French chemist, Marceline Bertrelot, synthesized carbon monoxide to formic acid. Formic acid has long been considered a chemical compound that has little interest in the chemical industry. In the late 1960s, significant quantities became available as a by-product of acetic acid production. It is now used as a preservative and antibacterial agent in animal feed.
What is formic acid?
Formic acid is named after ants that have high concentrations of the compound in their venom. In ants, formic acid is obtained from serine through the intermediate. Formic acid conjugate base, formate, is also widely found in nature. The method for measuring formic acid in body fluids, which is designed to determine the formate after methanol poisoning, is based on the reaction of the formate with the bacterial dehydrogenase formate.
Formic acid is flammable at a concentration of 85%. Formic acid contains 53 grams per liter of hydrogen at room temperature and atmospheric pressure, which is three and a half times what compressed hydrogen gas can get at 350 bar. Pure formic acid is a liquid with a flash point of +69 ° C, much higher than gasoline (-40 ° C) or ethanol (+13 ° C).
| formic acid | Product Name |
| Methanoic acid | Name Iupac Formic Acid |
| HCOOH | Chemical formula of formic acid |
| Colorless liquid | Appearance |
| 46.03 g/mol | Molecular weight |
| °C 8.4 | melting point |
| °C 100.8 | Boiling point |
| 1.23 g/cm3 | Density |
| 3.75 | Fixed separation |
| 1.57 cP | Viscosity at 26 ° C |
Properties of formic acid:
Formic acid is a colorless liquid with a pungent odor that can be mixed with water and most polar organic solvents and is partially soluble in hydrocarbons. In hydrocarbons and in the vapor phase, most single molecules are composed of dimers attached to hydrogen. Gaseous formic acid does not follow the ideal gas law due to its tendency to bind hydrogen. Solid formic acid, which can exist in both forms, is composed of an effective endless network of hydrogen bonded formic acid molecules. Formic acid forms a low-boiling azotrop with water. Liquid formic acid tends to super cool.
In 2009, worldwide production capacity of formic acid was 720,000 tons per year, which is almost evenly distributed between Europe and Asia, while production on all other continents was below 1,000 tons per year. Commercially available in solutions with different concentrations between 85 and 99% by weight.
Formic acid has the most chemical properties of other carboxylic acids. Solutions in alcohols spontaneously form esters due to their high acidity. Formic acid divides some of the reducing properties of aldehydes, solutions of gold, silver, and platinum into metals.
Natural production of formic acid:
In the wild, formic acid is found in most ants and in uninhabited bees of the genus Oxytrigona. Dark wood ants can spray formic acid on their prey or to defend the nest. The silkworm also sprays it if it is threatened by predators. Also found in nettle hairs. Formic acid is a natural component in the atmosphere that occurs due to forest release.
Formic acid production:
Formic acid production in Iran is done by different methods. When methanol and carbon monoxide are combined in the presence of a strong base, methyl formate is produced according to the chemical equation:
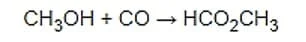
In industry, this reaction takes place in the high-pressure liquid phase. The usual reaction conditions are at a temperature of 80 ° C and a pressure of 40 atmospheres. The most widely used base is sodium methoxide. Hydrolysis of methyl formate produces formic acid:
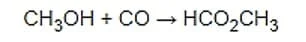
Efficient hydrolysis of methyl formate requires a large amount of water. Some routes are indirectly converted first to methyl formate with ammonia to formamide and then to hydrolysis with sulfuric acid:
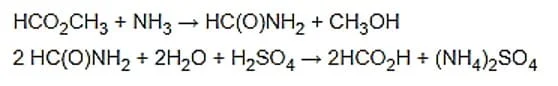
Another method is to produce formic acid by liquid-liquid extraction with an organic base from water. Significant amounts of formic acid are produced as a by-product in the manufacture of other chemicals.
Decomposition of formic acid:
Heat, especially acids, break down formic acid into carbon monoxide (CO) and water. In the presence of platinum, formic acid decomposes with the release of hydrogen and carbon dioxide.
Application of formic acid:
The uses of formic acid are very wide and include:
- As a preservative and antibacterial in animal feed
- To enhance lactic acid fermentation
- Suppression of butyric acid formation
- Reduce the loss of nutritional value in the fermentation process
- To preserve the winter feed of cattle
- To kill bacteria
- In leather production, including tanning
- In dyeing and finishing textiles
- As a coagulant in rubber production
- Instead of mineral acids for various cleaning products such as lime cleaners
- In flavors format esters
- To produce synthetic perfumes
- In beekeeping as a killer against ticks
- In the treatment of warts
- As a fuel cell
- As a mediator for the production of isobutanol from CO2 using microbes
- As the mobile phase in high performance HPLC liquid chromatography
- As a pH volatile modifier
- In capillary electrophoresis
- A resource for the Formille Group
- As a source of hydride ions
- As a source of hydrogen in the transfer of hydrogenation
- Production of carbon monoxide
- As a means of storing hydrogen
- As a food additive
- In the synthesis of drugs such as insulin and caffeine

